CONCERN
(D)
Potential for Harm - no one hurt, but risky conditions existed
Resident Rights
(Tag F0550)
Could have caused harm · This affected 1 resident
**NOTE- TERMS IN BRACKETS HAVE BEEN EDITED TO PROTECT CONFIDENTIALITY** Based on observations, interviews and record review, the facility failed to provide eating assistance in a dignified manner for ...
Read full inspector narrative →
**NOTE- TERMS IN BRACKETS HAVE BEEN EDITED TO PROTECT CONFIDENTIALITY** Based on observations, interviews and record review, the facility failed to provide eating assistance in a dignified manner for 2 of 2 sampled residents (Resident #103 and #276) observed for in-room dining, and failed to treat residents with dignity for 4 of 4 sampled residents observed by failing to provide a privacy pouch for an urinary bag (Resident #103); calling resident as a Feeder (Residents #82) failing to provide privacy during wound care (Resident #175); and failing to provide privacy to body parts and exposure resident (Resident #475).
The findings included:
Review of the facility policy provided by the Director of Nursing, untitled and undated documented, Dignity policy and procedure to ensure residents are treated with respect and individuality, promoting their self-esteem and well-being .respectful communication: address residents by their preferred name (not honey or sweetie .) .Maintaining privacy and confidentiality: ensure privacy during personal care activities .by using curtains or screens and minimizing unnecessary exposure .
1) Review of Resident #82's clinical record documented an admission to the facility on [DATE] with no readmissions. Resident #82's diagnoses included Vascular Dementia, Mild, Agitation and Anxiety Disorder. The resident's Minimum Data Set (MDS) quarterly assessment dated [DATE] documented a Brief Interview of Mental Status (BIMS) assessment was not conducted due to the resident is rarely/never understood. The assessment documented that the resident was dependent on the staff to eating.
On 03/17/25 at 12:44 PM, during in-room dining observation at the Masada Unit, Staff R, Certified Nursing Assistant (CNA) and Staff M, Unit Manager, were asked for Resident # 82's lunch intake. Staff M stated she would ask the aide. Staff M and Staff R both stated the resident is a feeder.
On 03/20/25 at 11:25 AM, an interview was conducted with Staff R, CNA who was apprised of calling Resident #82 a feeder on (03/17/25) Monday. Staff R stated No, I said they need assistance.
On 03/20/25 at 11:35 AM, an interview was conducted with Staff M, Unit Manager (UM), who was apprised of calling Resident #82 a feeder.
2) Review of Resident #103's clinical record documented an initial admission to the facility on [DATE]. Resident 103's diagnoses included Ventricular Premature Depolarization, Gallbladder Calculus and Hypertension.
Review of Resident #103's MDS Quarterly assessment dated 01/18/ documented a Brief Interview of Mental Status (BIMS) assessment was not conducted due to the resident is rarely/never understood. The assessment documented that the resident needed supervision/touching assistance during eating.
On 03/18/25 at 8:42 AM, observation revealed Resident #103 in bed and Staff P, CNA repositioning and setting up the resident for breakfast. An interview was conducted with Staff P who stated the resident feeds himself. The surveyor attempted to interview the resident, who kept his eyes open and fixed looking at the surveyor and did not answer any questions asked. At 8:43 AM, observation revealed Staff P left the resident's room.
On 03/18/25 at 9:06 AM, observation revealed Resident #103 sitting up in bed, asleep, eyes closed and his food tray across from him. Further observation revealed the food tray items were untouched. Furthermore, observation revealed Resident #103 did not have a staff cuing him to eat or assisting him to eat from 8:42 AM until 9:07 AM.
On 03/18/25 at 9:07 AM, observation revealed Staff M, UM entered Resident #103's room, and asked if he finished eating. Staff M repositioned a chair and started feeding the resident who was observed eating his breakfast as he was fed by Staff M.
On 03/19/25 at 9:22 AM, an interview was conducted with Staff M, UM, who stated that Resident #103 usually goes to the dining room and feeds himself and added that the aide probably did not know the resident. She further stated the aide should have come to the nurse and tell her that he was not eating. Staff M was apprised of the surveyor's concerns that the resident waited approximately 25 minutes to be fed. Staff M stated it is concerning.
3) Review of Resident #103's clinical record documented an initial admission to the facility on [DATE]. Resident's diagnoses included Ventricular Premature Depolarization, Cystostomy Status, Gallbladder Calculus and Hypertension.
Review of Resident #103's MDS Quarterly assessment dated 01/18/ documented a Brief Interview of Mental Status (BIMS) assessment was not conducted due to the resident is rarely/never understood. The assessment documented that the resident had an indwelling catheter (foley).
Review of Resident #103's care plan titled [resident's name] has a risk for injury/infection r/t (related to) presence of indwelling catheter secondary to a dx (diagnosis) of neurogenic bladder initiated on 10/11/24 with interventions to include Privacy bag/cover in place, initiated on 10/11/24.
On 03/18/25 at 8:42 AM, observation revealed Resident #103 in bed and Staff P, CNA repositioning and setting up the resident for breakfast. Observation revealed a urinary drainage bag with no privacy pouch. An interview was conducted with Staff P who stated the resident had a foley catheter. The surveyor attempted to interview the resident who kept his eyes open and fixed looking at the surveyor and did not answer any questions asked. At 8:43 AM, observation revealed Staff P left the resident's room and did not place a privacy pouch on the resident's urinary bag to provide privacy.
On 03/18/25 at 8:46 AM, observation revealed the Infection Preventionist and Staff M, UM, placing a cart with Personal Protective Equipment outside of Resident #103's room. An interview was conducted with Staff M and the Infection Preventionist who both stated the resident had a foley.
On 03/19/25 at 9:22 AM, an interview was conducted with Staff M, UM who was apprised that Resident #103 did not have a privacy pouch to cover the urinary drainage bag that was observed on 03/18/25.
4) Review of Resident #175's clinical record documented an admission to the facility on [DATE] with no readmissions. The resident's diagnoses included End Stage Renal Disease and Anxiety Disorder.
Review of Resident #175's MDS admission assessment in progress dated 03/13/25 documented a BIMS score of 13, indicating that the resident had no cognition impairment.
Review of Resident #175's care plan titled [resident's name] has a pressure ulcer to sacrum initiated on 03/06/25 documented intervention to include .Administer medications and treatments as ordered by the MD (Medical Doctor).
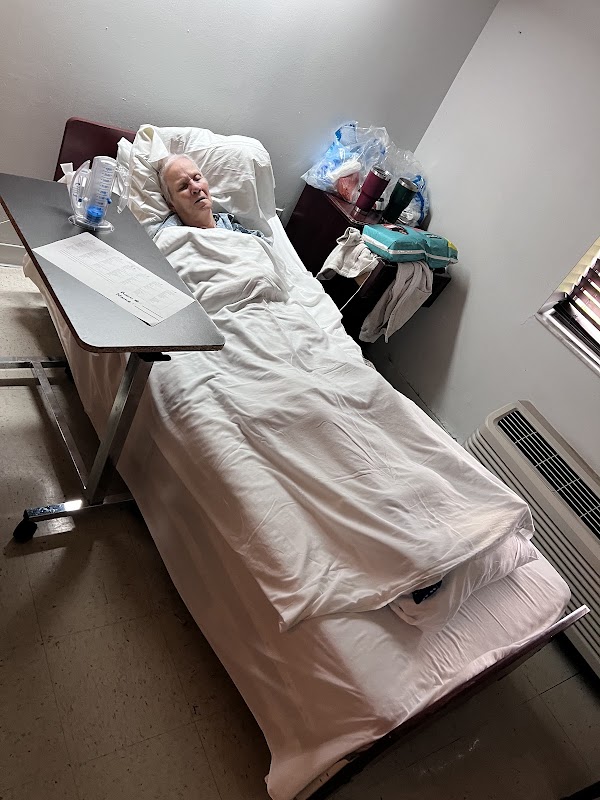
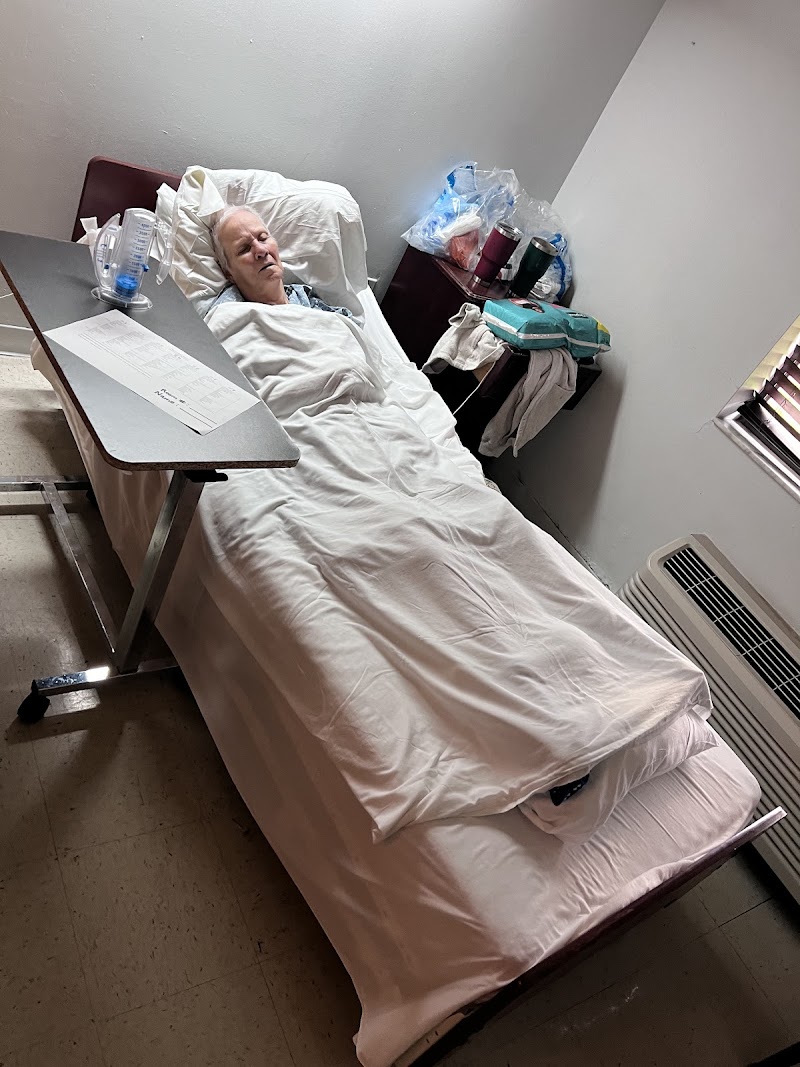
On 03/17/25 at 12:17 PM, observation revealed Resident #175's room door wide open, a treatment cart parked in front of the door, and the resident's privacy curtain halfway open (Photographic evidence). The surveyor knocked at the door and was allowed to enter the room. Staff I stated Staff J was doing the resident's wound care. The observation revealed Staff I, CNA and Staff J, Wound Care Nurse (WCN) next to the resident's bedside. The resident had his cover down and was exposing a foley tubing and his legs. Staff J stated she was finishing the resident's wound care. Observation then revealed Staff J and Staff I pull the cover sheet and blanket up.
On 03/20/25 at 2:36 PM, during an interview, the Director of Nursing was apprised of the findings.
5) A chart review revealed that Resident #275 was admitted to the facility on [DATE] with diagnoses of Alzheimer ' s and unspecific protein-calorie malnutrition. The admission MDS assessment dated [DATE] revealed Resident #275 has a Brief Interview of Mental Status score (BIMS) of 05, which is severely cognitively impaired. Section GG for eating showed Resident #275 needed partial to moderate assistance.
In an observation conducted on 03/17/25 at 12:34 PM, Resident #275 ' s roommate received his lunch tray. At 12:46 PM, Resident #275 still awaited his lunch tray. At 12:53 PM, which was 19 minutes later, Staff F, a Certified Nursing Assistant, came with the lunch tray for Resident #275. Closer observation showed that Resident #275 ' s roommate was done with his lunch meal.
In an interview conducted on 03/20/25 at 8:50 AM with Staff F, who stated all residents must be treated with dignity. The curtain and the door need to be closed when providing patient care and ensuring residents are not exposed. It is important not to call residents names or use the word feeders. Staff F further said during dining, you need to pass the meal trays one room at a time so that you do not have one resident eating while the other resident is not.
6) Record review for Resident #475 revealed the resident was admitted to the facility on [DATE] with diagnoses that included in part, the following: Chronic Obstructive Pulmonary Disease, Acute Respiratory Failure with Hypoxia and Gastrostomy Status. The resident did not have a completed Minimum Data Set at time of review.
On 03/17/25 at 11:10 AM an observation was made of Resident #475 lying on his side in bed with the bed covers off, his shorts unbuttoned and half off with a disposable brief partially exposed and a peg tube coming out from under his shirt and draped over the resident's side.
On 03/17/25 at 12:45 PM and 3:05 PM to 3:40 PM, the observation revealed Resident #475 with the door to the room open and full view from the hallway. The resident was lying in bed with no bed linens covering him while wearing only an adult brief and a shirt with his back to the door.
CONCERN
(D)
Potential for Harm - no one hurt, but risky conditions existed
Notification of Changes
(Tag F0580)
Could have caused harm · This affected 1 resident
**NOTE- TERMS IN BRACKETS HAVE BEEN EDITED TO PROTECT CONFIDENTIALITY** Based on interview and record review the facility failed to document notification of the resident or resident representative for...
Read full inspector narrative →
**NOTE- TERMS IN BRACKETS HAVE BEEN EDITED TO PROTECT CONFIDENTIALITY** Based on interview and record review the facility failed to document notification of the resident or resident representative for change in condition for 2 of 2 sampled residents reviewed for a change in condition (Resident #488 and Resident #53).
The findings included:
1) Record review for Resident #53 revealed the resident was admitted to the facility on [DATE] with diagnoses that included in part, the following: Dementia and Major Depressive Disorder. He was discharged to the hospital on 2/27/25. Review of the Minimum Data Set assessment dated [DATE] documented in Section C a Brief Interview of Mental Status score of 15, indicating intact cognition.
Review of the Nurses Notes for Resident #53 dated 02/27/25 documented: At approximately 9:10 AM a call was received from the doctor's office, due to the resident's vitals being unstable. Per physician, the resident was transferred to the hospital emergency room for further evaluation.
Further review of the medical record for Resident #53 revealed no evidence of a Change in Condition Evaluation was completed, no documentation of notification of resident representative or emergency contact being notified, and no documentation of the resident leaving for a physician's visit and/or with whom.
2) Record review for Resident #488 revealed the resident was admitted to the facility on [DATE] with diagnoses that included in part, the following: Ischemic Cardiomyopathy. Review of the Minimum Data Set assessment dated [DATE] documented in Section C a Brief Interview of Mental Status score of 15, indicating an intact cognition.
Review of the Nurses Notes for Resident #488 dated 3/11/25 included in part, the following: Observed the resident in bed awake and alert, however a little sluggish. Life-Vest in place. Head of bed elevated. Vitals signs taken. Oxygen saturation fluctuates from 89-94%. ARNP made aware, order for non-rebreather at 15 L/min and to transfer out to [name of hospital] Via 911. Call placed to 911.
Review of the Nurses Notes for Resident #488 dated 3/11/25 documented: At approximately 2:30 PM The resident was transferred to ER (Emergency Room) via 911.
Review of the Change in Condition Evaluation for Resident #488 dated 03/11/25 documented in Section 3, Review and Notify Section C Name of family/resident representative notified: was left blank.
Review of all documentation for Resident #488 on 03/11/25 revealed no evidence of any family present or notification.
During an interview conducted on 03/19/25 at 4:20 PM with Staff G Licensed Practical Nurse Unit/ Manager who was asked about a change in condition, she stated when a resident has change in condition, they will notify the family or the representative at the time of the change of condition. When asked if she documents who was notified, she said yes the family or representative or emergency contact, whomever they speak to or leave a message for. When asked if a resident was out of the facility attending a medical appointment and the physician was sending the resident to hospital directly, would they notify the family or emergency contact, she stated they would notify the family, representative or emergency contact. When asked about Resident #488 she said the daughter was in the facility at the time the resident was having the change in condition. She acknowledged she did not document that the daughter was present and was aware of the change in condition. When asked about Resident #53, she stated the wife was with the resident and since it happened at the doctor's office and they were sending the resident to the hospital, she did not contact the wife and thought she would be aware of the situation. She also acknowledged she did not document the change in condition evaluation for Resident #53.
CONCERN
(D)
Potential for Harm - no one hurt, but risky conditions existed
Safe Environment
(Tag F0584)
Could have caused harm · This affected 1 resident
**NOTE- TERMS IN BRACKETS HAVE BEEN EDITED TO PROTECT CONFIDENTIALITY** Based on observations and interviews, the facility failed to provide a safe, clean, comfortable and homelike environment for 9 o...
Read full inspector narrative →
**NOTE- TERMS IN BRACKETS HAVE BEEN EDITED TO PROTECT CONFIDENTIALITY** Based on observations and interviews, the facility failed to provide a safe, clean, comfortable and homelike environment for 9 of 64 rooms.
The findings included:
1). On 03/17/25 at 11:01 AM, an observation of room [ROOM NUMBER] revealed the flooring and wall behind the resident's bed were stained and the baseboard was in disrepair.
2). On 03/17/25 at 11:15 AM, an interview was conducted with Resident #16 who stated her privacy curtains needed to be washed. Observation revealed the residents' privacy curtain was stained. Further observation revealed the flooring was stained and the baseboard behind the bed was in disrepair.
3). On 03/17/25 at 11:20 AM, an observation revealed the bathroom light of room [ROOM NUMBER] was dim and blinking. The baseboard behind the resident's bed was in disrepair.
4). On 03/18/25 at 8:35 AM, an observation and interview with Resident #27 revealed her privacy curtain did not cover the window area. The resident further added that the curtain had been like that since she was moved to the room (Photographic evidence obtained).
5). On 03/17/25 at 12:12 PM, observation revealed room [ROOM NUMBER]'s wall outside the room door was in disrepair. Further observation inside of the room revealed the resident's dresser drawer with a broken piece of wood and a TV connected to a power strip.
6). On 03/17/25 at 11:40 AM, observation revealed room [ROOM NUMBER]'s baseboard behind the resident's bed and nightstand was in disrepair and the flooring was stained.
On 03/19/25 at 4:15 PM, an environmental tour was conducted with the Environmental Services Representative and the Housekeeping Director. The tour revealed the following:
7). room [ROOM NUMBER] revealed a strong urine-like odor in the bathroom. The room baseboards were blackened in various sections. The bathroom wall near the door was soft and the plaster was not smooth.
8). room [ROOM NUMBER] and 112 revealed a strong urine-like odor.
The Environmental Services Representative stated all of the room baseboards were previously painted over and added that the baseboard material is plastic and when they clean and buff the floor, the paint comes off. The Environmental Services Representative stated they have a plan to change all room baseboards and flooring and are awaiting on a tile delivery.
Upon interview on 03/19/25 at 4:35 PM, during the tour, the Environmental Services Representative acknowledged the environmental concerns that were identified on 03/17/25 and 03/19/25.
CONCERN
(D)
Potential for Harm - no one hurt, but risky conditions existed
Comprehensive Care Plan
(Tag F0656)
Could have caused harm · This affected 1 resident
**NOTE- TERMS IN BRACKETS HAVE BEEN EDITED TO PROTECT CONFIDENTIALITY** Based on observations, interviews and record review, the facility failed to initiate an activities care plan for 1 of 1 sampled ...
Read full inspector narrative →
**NOTE- TERMS IN BRACKETS HAVE BEEN EDITED TO PROTECT CONFIDENTIALITY** Based on observations, interviews and record review, the facility failed to initiate an activities care plan for 1 of 1 sampled resident reviewed for activities (Resident #12) and failed to initiate a wound care plan for 1 of 2 sampled residents reviewed for pressure ulcers (Resident #39).
The findings included:
Review of the facility's policy untitled, undated, provided by the Director of Nursing, documented Resident Activities policy and procedure ensures resident's rights to participate in activities to promote well-being and engagement .Individualized care planning .develop a comprehensive activity plan that includes a variety of activities, schedules and staff responsibilities .
1) Review of Resident #12's clinical record documented an initial admission to the facility on [DATE] and readmission [DATE]. The resident's diagnoses included Contracture, Left Hand, Contracture, Left Ankle, Age-Related Nuclear Cataract, Bilateral, Spastic Hemiplegia Affecting Left Nondominant Side, Epilepsy, Neuropathy, and Chronic Pain.
Review of Resident #12's Minimum Data Set (MDS) quarterly assessment dated [DATE] documented a Brief Interview of the Mental Status (BIMS) score of 15, indicating that the resident had no cognition impairment. The assessment documented under Functional Abilities and Goals that the resident needed extensive assistance/total assistance from the staff to complete the activities of daily living, does have upper extremities impairment and uses a wheelchair.
Review of Resident #12's MDS Annual assessment dated [DATE] documented a BIMS of 15, indicating that the resident have no cognitive impairment. The Activities section of the assessment documented the following:
*How important is it to you to have books, newspapers and magazines to read? Somewhat important.
*How important is it to you to listen to music you like? Not very important.
*How important is it to you to do things with groups of people? Not important at all.
*How important is it to you to do your favorites activities? Somewhat important.
*How important is it to you to go outside to get fresh air when the weather is good? Very important.
*How important is it to you to participate in religious services or practices? Very important.
Resident #12's clinical record lacked written evidence of an activities care plan developed.
On 03/17/25 at 1:03 PM, an interview was conducted with Resident #12 who stated she felt lonely in her room, could not remember short or long-term things. The resident stated she used to have a workbook, but it was lost when she was moved from another room and the facility staff couldn't find it. Resident #12 was asked if someone from activities comes to her room to do any type of activity and stated, 'No. The resident was asked if she would like to do some in-room activities and stated, Yes.
On 03/19/25 at 11:42 AM, an interview was conducted with the Activities Director (AD) who stated she has been working at the facility since 12/24. The AD was asked about Resident #12's Activities care plan and stated she did a care plan on 02/11/25. A side-by-side review with the AD of Resident # 12's IDT (Interdisciplinary Team) Care Conference Summary dated 02/11/25. The AD stated that it was the activities care plan.
On 03/19/25 at 12:20 PM, a side-by-side review of Resident # 12's active/current care plans was conducted with Staff N, MDS Coordinator and MDS Lead. They were asked for Resident #12's activities care plan, Staff N stated he did not see one. The MDS Lead stated the activities department was supposed to create an activities care plan. Staff N stated the Activities Department staff should have completed the care plan. The MDS Lead stated when they meet for care plan conferences, the IDT goes over the care plan and updates or creates a care plan.
On 03/20/25 at 3:45 PM, during an interview, the Director of Nursing and the Administrator were apprised of Resident #12's lack of a written care plan and the lack of documentation of activities provided. The Administrator acknowledged that if it is not documented it was not done.
2) Record review for Resident #39 revealed the resident was originally admitted to the facility on [DATE] and a most recent readmission on [DATE], with diagnoses that included in part, Heart Failure and Kidney Transplant Status. The Minimum Data Set assessment dated [DATE] documented in Section C, a Brief Interview of Mental Status score of 14, indicating a cognitive response.
Review of the Physician's Orders for Resident #39 revealed an order dated 03/13/25 as cleanse Right Heel wound with normal saline, pat dry, apply Betadine, cover with gauze, and wrap with Kerlix every day shift for wound.
Review of the Physician's Orders for Resident #39 revealed an order dated 03/13/25 for cleanse wound to Right Leg with normal saline, pat dry, apply Betadine, cover with dry protective dressing every day shift for wound.
Review of the Physician's Orders for Resident #39 revealed an order dated 03/18/24 for Enhanced Barrier Precautions for wound care.
Review of the Care Plans for Resident #39 revealed no care plan for the right heel or right leg wound, and no care plan for Enhanced Barrier Precautions.
On 03/18/25 at 8:55 AM, an observation was made of an already in-progress wound care, being provided for Resident #39, performed by Staff J, a Wound Care Licensed Practical Nurse, who was assisted by Staff I, a Certified Nursing Assistant (CNA). There were no Enhanced Barrier Precautions sign on the resident's door, and no isolation cart near the resident's door.
An interview was conducted on 03/20/25 at 12:50 PM with Staff N, MDS Coordinator, who stated he has been in his position for just under one year. When asked if he is responsible for creating care plans for the residents, he said yes, if they are nursing care plans. When asked when he would enter the care plan for a resident with a wound, he stated it would be the same day or the next day. When asked about Resident #39 he acknowledged there was no care plan for the right leg and right heel wound that was identified on 03/13/25. Additionally, he acknowledged there was no care plan for Enhanced Barrier Precautions for Resident #39.
An interview was conducted on 03/20/25 at 1:15 PM with Staff J Wound Care Licensed Practical Nurse who stated she has been with the facility for almost 1 year. When asked if she creates a care plan or enters orders for Enhanced Barrier Precautions when she enters an order for a new wound, she said no. She stated the MDS department will review her notes and create a care plan and the Infection Preventionist will review her notes and enter an order for Enhanced Barrier Precautions.
CONCERN
(D)
Potential for Harm - no one hurt, but risky conditions existed
ADL Care
(Tag F0677)
Could have caused harm · This affected 1 resident
**NOTE- TERMS IN BRACKETS HAVE BEEN EDITED TO PROTECT CONFIDENTIALITY** Based on observations, interviews, and record review, the facility failed to provide assistance during dining for 2 of 2 sampled...
Read full inspector narrative →
**NOTE- TERMS IN BRACKETS HAVE BEEN EDITED TO PROTECT CONFIDENTIALITY** Based on observations, interviews, and record review, the facility failed to provide assistance during dining for 2 of 2 sampled residents reviewed for Activities of Daily Living (ADL) (Resident #1 and Resident #276).
The findings included:
A review of the facility policy titled Activities of Daily Living Policy (undated), documented the following: Identify the specific needs and goals of each Resident, considering their individual preferences and abilities. Provide assistance with feeding as needed and ensure proper nutrition and hydration.
1) A chart review revealed Resident #1 was admitted to the facility on [DATE] with diagnoses of Rheumatoid Arthritis and Falls. The Minimum Data Set (MDS) assessment dated [DATE] revealed Resident #1 is severely impaired cognitively. Under section GG for eating, Resident #1 was coded as partial to moderate assistance during dining. This means the helper does less than half the effort. The helper lifts, holds, or supports trunks or limbs but provides less than half the effort.
In an observation conducted on 03/17/25 at 12:48 PM, the lunch tray came into Resident #1's room. The tray was noted with corn beef, parslied potatoes, buttered cabbage, Jello cubes, and a dinner roll. Continued observation at 12:55 PM revealed the tray was still untouched. At 1:02 PM, the tray was barely touched, with only a few bites from the buttered cabbage and no staff in the room. At 1:03 PM, Staff K, a Certified Nursing Assistant (CNA), took the lunch tray out of the room.
In an observation conducted on 03/17/25 at 5:32 PM, Resident #1 received her dinner tray, and no staff were noted in the room to assist the Resident with her dinner. At 5:45 PM, no staff were noted in the room to assist. Continued observation at 5:53 PM revealed Resident #1 ate about 10% of her dinner meal, with no staff in the room to assist. The dinner tray was noted with the following: Baked macaroni and cheese, stewed tomatoes, a brownie, a slice of bread, and a carton of milk. The carton of milk was noted unopened, and the brownie and slice of bread were still wrapped.
In an observation conducted on 03/19/25 at 8:32 AM, Resident #1 was eating her breakfast with no staff in the room to assist her. The tray was noted with the following: pancakes, scrambled eggs, hot cereal, juice and a carton of milk that was not poured into a cup. Closer observation showed that Resident #1 ate 20% of her breakfast meal.
In an interview conducted on 03/19/25 at 4:30 PM with Staff C, a Certified Nursing Assistant (CNA), stated that Resident #1 needed help during mealtimes, but now you only need to open the food containers and set up her tray, and she can eat independently.
2) A chart review revealed that Resident #275 was admitted to the facility on [DATE] with diagnoses of Alzheimer's and protein-calorie malnutrition. The admission MDS assessment dated [DATE] revealed Resident #275 has a Brief Interview of Mental Status score (BIMS) score of 05, which is severely cognitively impaired. Section GG for eating showed Resident #275 needed partial to moderate assistance.
In an observation conducted on 03/17/25 at 11:00 AM, Resident #275 was still in the room with his breakfast tray, and there were no staff in the room. The meal ticket noted the following: hot cereal, Western egg baked, soft white toast, fruit of the day, juice, and milk. Closer observation showed Resident #275 ate about 30% of his breakfast meal.
In an observation conducted on 03/17/25 at 12:53 PM, Staff F (CNA) came with the lunch tray for Resident #275. She sat down near the resident and started feeding him his lunch meal. In this observation, Staff F stated Resident #275 can eat independently, but some days, he cannot. He needs pushing and encouragement to eat his meals.
In an interview conducted on 03/17/25 at 5:40 PM with Resident #275's family member, he stated Resident #275 needs help and encouragement with all his meals.
An interview conducted on 03/20/25 at 8:34 AM with Staff D, Minimum Data Set Lead, who reported partial to moderate assistance during dining, means that residents can feed themselves but need some assistance. The resident needs observation during mealtimes and assistance completing their meals.
CONCERN
(D)
Potential for Harm - no one hurt, but risky conditions existed
Deficiency F0679
(Tag F0679)
Could have caused harm · This affected 1 resident
**NOTE- TERMS IN BRACKETS HAVE BEEN EDITED TO PROTECT CONFIDENTIALITY** Based on observation, interviews and record review, the facility failed to provide an ongoing activities program to support resi...
Read full inspector narrative →
**NOTE- TERMS IN BRACKETS HAVE BEEN EDITED TO PROTECT CONFIDENTIALITY** Based on observation, interviews and record review, the facility failed to provide an ongoing activities program to support resident's preferences for 1 of 1 sampled resident for Activities (Resident #12).
The findings included:
Review of the facility's policy untitled, undated, provided by the Director of Nursing documented Resident Activities policy and procedure ensures resident's rights to participate in activities to promote well-being and engagement .Individualized care planning .develop a comprehensive activity plan that includes a variety of activities, schedules and staff responsibilities .
Review of Resident #12's clinical record documented an initial admission to the facility on [DATE] and readmission [DATE]. The resident's diagnoses included Contracture, Left Hand, Contracture, Left Ankle, Age-Related Nuclear Cataract, Bilateral, Spastic Hemiplegia Affecting Left Nondominant Side, Epilepsy, Neuropathy, and Chronic Pain.
Review of Resident #12's Minimum Data Set (MDS) quarterly assessment dated [DATE] documented a Brief Interview of the Mental Status (BIMS) score of 15 indicating that the resident had no cognition impairment. The assessment documented under Functional Abilities and Goals that the resident needed extensive assistance/ total assistance from the staff to complete the activities of daily living, does have upper extremities impairment and uses a wheelchair.
Review of Resident #12's MDS Annual assessment dated [DATE] documented a BIMS of 15, indicating that the resident had no cognitive impairment. The resident's Activities section of the assessment documented the following:
*How important is it to you to have books, newspapers and magazines to read? Somewhat important.
*How important is it to you to listen to music you like? Not very important.
*How important is it to you to do things with groups of people? Not important at all.
*How important is it to you to do your favorites activities? Somewhat important.
*How important is it to you to go outside to get fresh air when the weather is good? Very important.
*How important is it to you to participate in religious services or practices? Very important.
Resident #12's clinical record lacked written evidence of an activities care plan developed.
On 03/17/25 at 1:03 PM, an interview was conducted with Resident #12 who stated she felt lonely in her room, could not remember short or long term things. The resident was asked if she had an I-Pad, and replied she used to have a workbook and it was lost when she was moved from another room, the facility staff couldn't find it. The resident stated she was out of bed on Wednesday, it was her choice because her legs swell up and in pain. Resident #12 was asked if someone from activities comes to her room to do any type of activity, and she stated 'No. The resident was asked if she would like to do some in-room activities, and she stated Yes.
On 03/18/25 at 12:45 PM, observations revealed Resident #12 in bed, talking to her roommate.
On 03/19/25 11:42 AM, an interview was conducted with the Activities Director (AD) who stated she had been working at the facility since 12/24. The AD stated she does 1:1 in room activities, walks the units daily and knows who is in bed and who is not. She asks the residents if they want company, sometimes bring the coloring and crafts, talk and read to them. The AD was asked if she keeps a record of activities provided to the resident and stated she did not do or keep a lot of in-room activities, added she was supposed to but got side-tracked and did not do it. The AD stated she goes to do room visit 1:1 once a week and sometimes pops up twice a week. The AD stated she had two Activities Assistant always and three on Wednesday, Thursday and Fridays and two on the weekends. The AD was asked about Resident # 12's activities and stated she did her makeup three (3) times a week last week, and added she mostly reads and sits to talk with her because she likes company. The AD added the resident cries because of pain, likes the makeup, brings her to music events, and added the resident gets visits from friends from church every day. The AD was asked if she brings magazines or anything like that to the resident and stated she does not bring magazines because the resident had not asked for it. The AD stated she asked the resident what she likes and offered coloring. The AD stated the department had an I-Pad, but she had not offered it to Resident #12. The AD was asked to submit written evidence of 1:1 activities for Resident #12 and stated she does not document 1:1 visits or the activities provided for Resident # 12.
On 03/19/25 at 12:04 PM, a joint visit with the AD and Resident #12 was conducted. Resident #12 was up in a wheelchair. The AD asked the resident about her make up, the resident replied, you only had done it once, honey.
On 03/19/25 at 12:20 PM, a side by side review of Resident #12's active/current care plans was conducted with Staff N, MDS Coordinator and MDS Lead. The MDS Lead stated when they meet for care plan conference, the IDT goes over care plans and updates or create a care plan.
On 03/20/25 at 3:45 PM, during an interview, the Director of Nursing and the Administrator were apprised of Resident #12's lack of a written care plan and the lack of documentation of activities provided. The Administrator acknowledged that if it is not documented, t was not done.
CONCERN
(D)
Potential for Harm - no one hurt, but risky conditions existed
Quality of Care
(Tag F0684)
Could have caused harm · This affected 1 resident
**NOTE- TERMS IN BRACKETS HAVE BEEN EDITED TO PROTECT CONFIDENTIALITY** 3) Review of Resident #46's clinical record documented an admission to the facility on [DATE] with a readmission on [DATE]. The ...
Read full inspector narrative →
**NOTE- TERMS IN BRACKETS HAVE BEEN EDITED TO PROTECT CONFIDENTIALITY** 3) Review of Resident #46's clinical record documented an admission to the facility on [DATE] with a readmission on [DATE]. The resident's diagnoses included Dementia Disturbance, Aphasia Following other Cerebrovascular Disease, Trochanteric Fracture of Left Femur, Subsequent Encounter for Closed Fracture with Routine Healing, Presence of Left Artificial Hip Joint, Aftercare Following Joint Replacement Surgery, and Need for Assistance with Personal Care.
Review of Resident #46's Minimum Data Set (MDS) 5 days-admission assessment dated [DATE], documented a Brief Interview of the Mental Status (BIMS) was not conducted due to resident is rarely/never understood indicating the resident had severe cognition impairment. The assessment documented under Functional Abilities and Goals; the resident was dependent on the staff to complete the activities of daily living.
Review of Resident #46's care plan titled, [resident name] is at risk for complications related to left hip fracture initiated on 02/27/25 with interventions to include encourage and assist the resident with the use of adaptive equipment as indicated .
Review of Resident #46's physician order dated 02/27/25 documented, Abduction Pillow while in bed every shift.
Review of Resident #46's admission Notes dated 02/26/25 documented, .Primary diagnosis Left Hip fracture .Skin dry and warm to touch. Dry dressing noted to Left hip (Surgical site) . Safety and comfort measures maintained. Bed placed in low position with call light in reach.
On 03/17/25 at 11:33 AM, observation revealed Resident # 46 in bed, facial grimacing, and Staff BB, CNA was at the bedside. The surveyor attempted to interview the resident, who did not answer the questions asked. An interview was conducted with Staff BB who stated the resident is out of bed sometimes and gets pain medication. Further observation did not reveal the resident had an abduction pillow.
On 03/18/25 at 12:43 PM, observation revealed Resident #46 in bed being fed by Staff E, Licensed Practical Nurse (LPN). An interview was conducted with Staff E who stated Resident #46 fell last month and had a fracture, but did not know the details. Observation revealed the resident did not have an abduction pillow.
On 03/19/25 at 8:10 AM, observations revealed Resident #46 in a low position bed, moaning, lying down on her left side. Observation revealed the resident did not have an abduction pillow.
On 03/19/25 at 08:28 AM, observation revealed Resident # 46 in bed being fed by Staff O, CNA. The resident said hello and started to cry, and stated she had pain, unable to tell location. Observation revealed the resident did not have an abduction pillow.
On 03/19/25 at 9:00 AM, an interview was conducted with Staff BB, CNA. She stated she took care of Resident #46 on 03/17/25, did the personal care by herself but asked for help when she was ready to turn her. Staff BB stated she used regular pillows when repositioning the resident.
On 03/19/25 at 3:03 PM, a joint interview was conducted with Staff N, MDS Coordinator and MDS Lead. The MDS Lead was asked for Resident #46's Abduction pillow and stated the care plan was updated on 02/27/25, with an intervention to include the use of adaptive equipment. Consequently, a side-by-side observation was conducted of Resident #46's closet with the MDS Lead Staff M, Unit Manager. The observation revealed no abduction pillow in the resident's room. Staff M stated the resident brought the abduction pillow with her from the hospital (02/26/25).
On 03/19/25 at 3:25 PM, an interview was conducted with Staff S, CNA who stated she works the 3-11 shift. Staff S was asked what kind of pillow she used with Resident #46 while she was in bed and stated regular pillows. Staff S was asked if she had used a special pillow with the resident and stated she had not seen one in her room.
On 03/19/25 at 3:27 PM, an interview was conducted with Staff Q, CNA who stated she works the 3-11 shift. Staff S was asked what kind of pillow she used with Resident #46 while she was in bed and stated regular pillows on her back and left heel. Staff Q was asked if she had used a special pillow with the resident and she stated she had not.
On 03/19/25 at 3:46 PM, during an interview, Staff M, Unit Manager stated she was not aware that Resident #46 did not have the abduction pillow.
Based on record review, observation and interview, the facility failed to ensure residents receive treatment and care in accordance with professional standards of practice for 3 of 31 sampled residents including a Pleur-X (a type of chest tube) being drained as ordered (Resident #73) medications being administered in a timely manner as ordered (Residents #73 and #481); and failure to ensure a resident had an Abduction Pillow in place, as ordered by the physician (Resident #46)
The findings included:
1) Record review for Resident #73 revealed the resident was admitted to the facility on [DATE] with diagnoses that included, in part, the following: Malignant Neoplasm of Unspecified Part of Unspecified Bronchus or Lung, Pulmonary Embolism and Depression. Review of the Minimum Data Set (MDS) assessment dated [DATE] documented in Section C a Brief Interview of Mental Status (BIMS) score of 15, indicating a cognitive response.
Review of the Physician's Orders for Resident #73 revealed an order dated 03/05/25 for Drain Pleur-X every day shift every Monday, Wednesday, Friday and as needed.
Review of the Physician's Orders for Resident #73 revealed in part the following orders:
-An order dated 02/04/25 for Albuterol Sulfate Inhalation Nebulization Solution 1.25mg/3ml inhale orally via nebulizer every 4 hours for Shortness of Breath.
-An order dated 03/15/25 for Prednisone 10mg give 1 tablet by mouth one time a day for wheezing.
An order dated 03/21/25 for Lasix 40mg give 1 tablet by mouth one time a day for Edema hold for Systolic Blood Pressure less than 100.
-An order dated 02/21/25 for Eliquis 2.5mg give 1 tablet by mouth two times a day for Prevention of DVT/PE (Deep Vein Thrombosis/Pulmonary Embolism).
-An order dated 02/03/25 for Ipratropium Bromide HFA Inhalation Aerosol 17 mcg/act 2 puff inhale orally four times a day for Shortness of Breath.
Review of the Medication Administration Audit Report for Resident #73 revealed the following:
-On 03/18/25 Albuterol Sulfate Inhalation Nebulization Solution 1.25mg/3ml scheduled for 8:00 AM was administered at 10:42 AM
-On 03/18/25 Prednisone 10mg scheduled for 8:00 AM was administered at 10:33 AM
-On 03/18/25 Lasix 40mg scheduled for 9:00 AM was administered at 10:42 AM
-On 03/18/25 Eliquis 2.5mg scheduled for 9:00 AM was administered 10:33 AM
-On 03/18/25 Midodrine 5mg scheduled for 9:00 AM was administered at 10:33 AM
-On 03/18/25 Ipratropium Bromide scheduled for 9:00 AM was administered at 10:32 AM
-On 03/18/25 Ipratropium Bromide scheduled for 12:00 PM was administered at 11:04 AM
-On 03/18/25 albuterol sulfate Inhalation Nebulization Solution 1.25mg/3ml scheduled for 12:00 PM was administered at 11:04 AM.
In summary the Medication Administration Audit Report for Resident #73 revealed 6 medications were given late by as much as an hour and forty-two minutes. Additionally, nebulizer breathing medications were not administered 4 hours apart as ordered, they were administered 32 minutes apart.
Review of the Medication Administration Record (MAR) for Resident #73 for the month of March revealed no documentation of the Pleur-X being drained on 03/17/25.
Review of the Nurse's notes for Resident #73 for 03/17/25 revealed no documentation of Pleur-X being drained or not being drained.
Review of the Care Plan for Resident #73 dated 02/23/25 with a focus on Management of Pleur-X drainage and a goal of the resident will have no complications related to Pleur-X drainage. The interventions included the following: Staff will maintain appropriate function of chest tube. Monitor for chest pain. Monitor for signs and symptoms of infection, leakage or malfunction and report to Medical Doctor. Verify the appropriate equipment is at the bedside.
During an interview conducted on 03/17/25 at 11:14 AM Resident #73 stated the nurse did not give him his Eliquis this morning when she came into the room at 9:00 AM this morning. He said this has been an issue in this facility with not getting medications, sometimes you do get them and sometimes they are very late.
During an interview conducted on 03/18/25 at 10:00 AM with Resident #73 who stated he has a chest tube that needs to be drained 3 times a week and staff did not drain his tube yesterday all day. He was very upset and said, This is a serious life and death issue. In this interview, it was quite evident the resident was angry, irrigated and anxious. He then said to the Surveyor that he was concerned for his health.
During an interview conducted on 03/18/24 at 10:30 AM with Staff L Licensed Practical Nurse (LPN) who was asked if she was aware Resident #73 did not have his Pleur-X drained yesterday, she stated she was not aware, she did not have the resident yesterday and she was not given any information in report about his Pleur-X not being drained.
During an interview conducted on 03/18/25 at 10:33 AM with Staff G Licensed Practical Nurse Unit Manager who said she worked yesterday and was unaware of Resident #73 not having his Pleur-X drained yesterday. She acknowledged there was no documentation of the Pleur-X being drained and there was no progress note to indicate reason why not drained or the physician being notified.
During an interview conducted on 03/18/25 at 10:45 AM with Resident #73, it was noted that he was visibly upset and anxious talking fast with a raised voice and stated his drain was not drained yesterday as it should have been. He stated that he had surgery to have the drain inserted and he desperately needs the fluid drained as the fluid builds up and causes pressure in his chest. He stated the drainage process is painful and he needs pain medication for the pain until his lung goes back to being fully inflated.
During an interview conducted on 03/18/25 at 5:24 PM with the Attending Physician for Resident #73 who was asked what is the reason Resident #73 has a Pleur-X, the Attending Physician stated it is usually because fluid keeps on reaccumulating, so a catheter with a valve was put in and it is not difficult to drain. The Attending Physician went on to say it can stay in for a long time. When asked about the importance of it being drained as ordered, he said when it gets full you drain it or if the resident is out of breath. It was clarified with the Attending Physician that the Pleur-X catheter is not connected to any drainage type of collection. The Attending Physician stated he was driving and does not remember every detail of every patient and stated it would be the orders from the pulmonologist that would be followed. It was then clarified with the Attending Physician that the order was given by him. When asked what happens if it is not drained as ordered he stated it is not necessary to drain it, but if fluid is accumulating the resident would be out of breath and would need to be drained. When asked if the Pleur-X was not drained should he be informed he said he should be informed but could not recall if he was informed if the Pleur-X had not been drained.
During an interview conducted on 03/19/25 at 10:00 AM with Resident #73 who was asked how he felt, he said thank you for intervening on my behalf, things really started happening. They drained my Pleur-X yesterday and he feels much better and feels confident they will not let it happen again.
2) Record review for Resident #481 revealed the resident was admitted to the facility on [DATE] with diagnoses that included in part, the following: Aftercare Following Joint Replacement Surgery, Fracture of Specified Part of Neck of Right Femur Subsequent Encounter for Closed Fracture with Routine Healing, Unspecified Atrial Fibrillation, Essential (Primary) Hypertension. Review of the MDS for Resident #481 dated 02/18/25 documented in Section C a BIMS score of 15, indicating a cognitive response.
Review of the Physician's Orders for Resident #481 revealed in part, the following:
An order dated 02/24/25 for Diltiazem HCl oral tablet 120 mg give 1 tablet by mouth every 12 hours for Hypertension.
An order dated 02/24/25 for Sotalol HCl oral tablet 120 mg give 1 tablet by mouth every 12 hours for Arrhythmia.
Review of the Medication Administration Audit Report for Resident #481 revealed the following:
-On 03/13/25 Diltiazem 120 mg was scheduled to be administrated at 9:00 PM and was not given until 10:15 PM.
-On 03/13/25 Sotalol 120 mg was scheduled to be administrated at 9:00 PM and was not given until 10:14 PM.
-On 03/15/25 Diltiazem 120 mg was scheduled to be administrated at 9:00 PM and was not given until 03/16/25 at 12:29 AM.
-On 03/15/25 Sotalol 120 mg was scheduled to be administrated at 9:00 PM and was not given until 03/16/25 at 12:28 AM.
-On 03/16/25 Diltiazem 120 mg was scheduled to be administrated at 9:00 PM and was not given until 10:51 PM.
-On 03/16/25 Sotalol 120 mg was scheduled to be administrated at 9:00 PM and was not given until 10:52 PM.
-On 03/17/25 Diltiazem 120 mg was scheduled to be administrated at 9:00 PM and was not given until 11:02 PM.
-On 03/17/25 Sotalol 120 mg was scheduled to be administrated at 9:00 PM and was not given until 10:53 PM.
In summary the Medication Administration Audit Report for Resident #481 revealed cardiac medications were administered late on 8 occasions as late as 2 hours and 29 minutes.
During an interview conducted on 03/17/25 at 1:05 PM with Staff A, Registered Nurse, who was asked when medication administration is considered late or early, she stated they have an hour before and an hour after the medication scheduled time to give the medication.
During an interview conducted on 03/18/25 10:43 AM with Resident #481, she stated they give her heart medications late sometimes, more than two hours. When asked if she knew the names of her medications, she said Diltiazem and Sotalol.
During an interview conducted on 03/18/25 at 10:00 AM with the Consultant Pharmacist who was asked about medications being given late, such as Diltiazem and Sotalol for Resident #481, she stated some could be detrimental but not life threatening. When asked about the Albuterol Sulfate and Ipratropium Bromide inhalation medications being given close together (less than 30 minutes) she stated it could be detrimental but not life threatening.
CONCERN
(D)
Potential for Harm - no one hurt, but risky conditions existed
Tube Feeding
(Tag F0693)
Could have caused harm · This affected 1 resident
**NOTE- TERMS IN BRACKETS HAVE BEEN EDITED TO PROTECT CONFIDENTIALITY** 2) A record review revealed Resident #109 was readmitted to the facility on [DATE] at 5:32 PM with diagnoses of Dysphagia and Un...
Read full inspector narrative →
**NOTE- TERMS IN BRACKETS HAVE BEEN EDITED TO PROTECT CONFIDENTIALITY** 2) A record review revealed Resident #109 was readmitted to the facility on [DATE] at 5:32 PM with diagnoses of Dysphagia and Unspecific Protein-Calorie Malnutrition. The Significant Change Minimum Data Set assessment dated [DATE] showed Resident #109 was severely cognitively impaired.
A review of the Dietitian evaluation dated 2/3/25 revealed the Resident had an admission weight of 108.6 pounds. His Ideal day weight was noted at 160 pounds, and he was readmitted with a decline in weight.
The Physician's orders showed an order for tube feeding Jevity 1.5 (tube feeding formulary) at 50 milliliters (ml) an hour for 20 hours off at 8:00 AM and starting at 12:00 PM, which was placed on 2/3/25 at 3:00 PM. This was almost 22 hours after Resident #109 was admitted .
In an observation conducted on 03/17/25 at 11:24 AM, Resident #109 was sitting in a chair with the tube feeding not running. Continued observation at 12:34 PM revealed a bottle of tube feeding Jevity 1.5 with a start date of 03/17/25 at 12:00 PM, running at 50 milliliters (ml) an hour. The tube feeding was at the 1000 ml mark out of a 1000 ml capacity bottle.
In an observation conducted on 03/17/25 at 3:43 PM, Resident #109 was noted in a chair with the same tube feeding bag running at 50 ml an hour. The tube feeding bag was noted at the 950 ml mark out of a 1000 ml capacity bottle. This showed that only 50 ml of formulary was administered instead of about 200 ml of formulary.
In an observation conducted on 03/17/25 at 5:00 PM, Resident #109 was noted in a chair with the same tube feeding bag running at 50 ml an hour. The tube feeding bag was noted at 900 ml mark out of a 1000 ml capacity bottle. This showed that only 100ml of formulary was administered instead of about 250 ml of formulary. The tube feeding bottle should have been at the 750 ml mark after 5 hours.
In an interview conducted on 03/17/25 at 5:36 PM, Staff E, Licensed Practical Nurse, she stated started Resident #109's tube feeding at noon today and that it has been running continuously for the last 5 hours at 50 ml an hour. She further said, Resident #109 was tolerating his tube feeding well.
In an observation conducted on 03/18/25 at 12:45 PM, Resident #109 was noted in the chair with the tube feeding Jevity 1.5 at 50 ml an hour, which started on 03/18/25 at noon time. The tube feeding was noted at the 1000 ml level out of a 1000 ml capacity bottle. Continued observation at 3:49 PM, revealed that same tube feeding bottle which was at the 900 ml level out of 1000 ml capacity bottle. This showed that only 100 ml of formulary was administered instead of about 200 ml as per order.
A review of the care plan dated 02/10/25 documented to provide the tube feeding Jevity 1.5 as ordered.
In an interview conducted on 03/20/25 at 8:05 AM with the facility ' s Clinical Dietitian, she stated Resident #109 tube feeding should be running at 50 ml an hour for 20 hours to meet nutritional needs. When asked about the observation done by this Surveyor on 03/17/25, she acknowledged that the tube feeding should have been around the 750 ml mark at 5:00 PM. You may see a 10-20 ml variance in the tube feeding level, but no more than that. The Clinical Dietitian said that a variance of 100-200 ml was too much, especially if Resident #109 was tolerating his tube feeding.
Based on observations, interviews and record reviews, the facility failed to initiate tube feeding in a timely manner for 1 of 2 sampled residents reviewed for tube feeding (Resident #475) and failed to follow physician's orders for tube feeding for 2 of 2 sampled residents reviewed for tube feeding (Residents #475 and Resident #109).
The findings included:
1) Record review for Resident #475 revealed the resident was admitted to the facility on [DATE] at 6:00 PM with diagnoses that included in part, the following: Chronic Obstructive Pulmonary Disease, Acute Respiratory Failure with Hypoxia and Gastrostomy Status. The resident did not have a completed Minimum Data Set at time of review.
Review of the Physician's Orders for Resident #475 revealed in part, the following orders:
An order dated 03/17/25 at 2:00 PM Enteral Feed Order every 4 hours Tube Feeding Formula Jevity. Administer 240 ml bolus feeding every 4 hours. The order was discontinued on 03/17/25 at 2:11 PM.
An order dated 03/17/25 at 5:00 PM for Enteral Feed Order five times a day Tube Feeding Formula Jevity 1.5. Administer 237 ml bolus feeding every 5 cans QD every day. Flush with 120 ml (water) before and after each feeding.
An order dated 03/17/25 to check for skin integrity under the abdominal binder every shift.
Review of the Care Plan for Resident #475 dated 03/17/24 with a focus on the resident requires tube feeding related to Aspiration and Dysphagia. The goals were for resident to maintain adequate nutritional and hydration status and to remain free of side effects or complications related to tube feeding through review date. The interventions included in part, the following: Follow physician orders regarding nutrition order and flushes.
On 03/17/25 at 11:10 AM, an observation was made of Resident # 475 lying on his side in bed with the covers off, and what appeared to be a peg tube coming out from under his shirt and draped over the resident's side.
On 03/17/25 at 5:02 PM an observation was made of Staff A Registered Nurse (RN) administering tube feeding for Resident #475. Staff A RN applied a gown, entered the resident's room, washed her hands, applied gloves, touched the privacy curtain, and the bed control, then removed her gloves, washed her hands and applied gloves. The end of the PEG tube (type of feeding tube) had no cover or cap and was just clamped off. The resident did not have an abdominal binder on. Staff A RN checked for residual and there was none. The resident kept repeating Is this my food am I finally getting some food. The resident was also asking about pain medication. Staff A, RN stated he does not have any pain medication ordered, and she will have to call the doctor. Staff A RN poured Jevity 1.5 (formulary type) tube feeding from a closed system bottle that was opened and at the 450 mark and was dated 03/17/25 but had no time the bottle was opened.
During an interview conducted on 03/17/25 at 11:10 AM with Resident #475 he said his stomach hurts and he is hungry and hasn't eaten in days.
During an interview conducted on 03/17/25 at 04:20 PM with Staff A RN who was asked if Resident #475 had received any tube feeding today, she said yes, she gave him tube feeding at 3:02 PM today.
During an interview conducted on 03/17/25 at 5:20 PM with Staff A RN who was asked about the tube feeding being provided, she said they do not have cans, so they pour it from the larger bottle. When asked if Resident #475 has an order for nothing by mouth, she acknowledged he does. When asked if a resident comes in with a PEG tube and has no orders for tube feeding or a diet, what they do, she stated they would look at the hospital paperwork to see what the resident was receiving and then call the physician within two hours to get an order.
During an interview conducted on 03/20/25 at 10:00 AM with Staff G Licensed Practical Nurse Unit Manager who was asked about a resident who is admitted with a PEG tube and no tube feeding orders and no diet, she said the nurse would get the order from the physician within two hours. She checks the chart the next day as the Unit Manager to ensure all orders are in place. When asked about Resident #475 she acknowledged the resident was admitted to the facility on [DATE] at 6:00 PM and did not have an order for tube feeding until 03/17/25 at 2:00 PM.
CONCERN
(D)
Potential for Harm - no one hurt, but risky conditions existed
Pharmacy Services
(Tag F0755)
Could have caused harm · This affected 1 resident
**NOTE- TERMS IN BRACKETS HAVE BEEN EDITED TO PROTECT CONFIDENTIALITY** Based on interview and record review the facility failed to provide pharmaceutical services including procedures that assure the...
Read full inspector narrative →
**NOTE- TERMS IN BRACKETS HAVE BEEN EDITED TO PROTECT CONFIDENTIALITY** Based on interview and record review the facility failed to provide pharmaceutical services including procedures that assure the accurate dispensing and administrating of all drugs and ensure a system of records of administering all controlled drugs in sufficient detail to enable an accurate reconciliation and that drug records are in order and an account of all controlled drugs is maintained for 3 of 8 sampled residents reviewed for controlled drugs (Resident #487, #28, and 82).
The findings included:
1) Record review for Resident #487 revealed the resident was admitted to the facility on [DATE] with most recent readmission on [DATE] with diagnosis that included in part the following: Chronic Obstructive Pulmonary Disease and Essential (Primary) Hypertension. The Minimum Data Set (MDS) assessment dated [DATE] documented in Section C a Brief Interview of Mental Status (BIMS) could not be completed due to the resident is rarely/never understood.
Review of the Physician's Orders for Resident #487 revealed an order dated 02/24/25 for Valium Oral Tablet 5 MG (Diazepam) give 1 tablet by mouth every 4 hours as needed for Agitation for 14 Days.
Review of the Medication Monitoring/Control Record for Resident #487 Diazepam 5mg documented 03/13/25 at 2:29 PM the medication was removed from the med cart.
Review of the Medication Administration Record (MAR) for Resident #487 for the month of March 2025 revealed no documentation of Valium (Diazepam) 5mg being administered.
In summary the Valium (Diazepam) 5mg for Resident #487 was signed on the Medication Monitoring/Control Record as removed from the med cart but not documented as being administered on the resident's Medication Administration Record.
2) Record review for Resident #28 revealed the resident was admitted to the facility on [DATE] with diagnoses that included in part the following: Displaced Intertrochanteric Fracture of Right Femur, Subsequent Encounter for Closed Fracture with Routine Healing. The MDS assessment dated [DATE] documented in Section C, a BIMS (Brief Interview for Mental Status) score of 13, indicating a cognitive response.
Review of the Physician's Orders for Resident #28 revealed an order dated 04/09/24 for Oxycodone HCl Capsule 5mg give 1 capsule by mouth every 6 hours, as needed for moderate to severe pain.
Review of the Medication Monitoring/Control Record for Resident #28 Oxycodone 5mg documented on 02/14/25 at 4:57 PM the medication was removed from the med cart.
Review of the MAR for Resident #28 for the month of February 2025 revealed no documentation of Oxycodone 5mg being administered.
In summary the Oxycodone 5mg for Resident #28 was signed on the Medication Monitoring/Control Record as removed from the cart but not documented as being administered on the resident's Medication Administration Record.
3) Record review for Resident #82 revealed the resident was admitted on [DATE] with diagnoses that included in part the following: Cerebral Atherosclerosis and Vascular Dementia Mild with Agitation. The MDS dated [DATE] Documented in Section C, a BIMS was not performed due to the resident is rarely/never understood.
Review of the Physician's Orders for Resident #82 revealed an order dated 12/25/24 for Tramadol HCl Tablet 50 mg give 1 tablet by mouth every 8 hours as needed for moderate and severe pain.
Review of the Medication Monitoring/Control Record for Resident #82 for Tramadol 50mg revealed no documentation of the med being signed out as removed from the med cart.
Review of the Medication Administration Record for Resident #82 documented the Tramadol 50mg was administered on 03/16/25 at 12:00 AM and the Medication.
In summary the Tramadol 50 mg for Resident #82 was documented as administered but not signed out on the Medication Monitoring/Control Record as removed from the med cart.
During an interview conducted on 03/20/25 at 12:45 PM with the DON (Director of Nursing) who was asked who completes the monitoring or auditing of the medication reconciliation of controlled substances, she stated the Unit Managers does.
During an interview conducted on 03/20/25 at 1:05 PM with Staff G -Licensed Practical Nurse Unit Manager who said she does the audit of the controlled medication by checking the Medication Monitoring/Control Record to make sure all entries have a signature, and it matches the residents Medication Administration Record. She is supposed to do this once a week, but she does it usually three times a week.
CONCERN
(D)
Potential for Harm - no one hurt, but risky conditions existed
Deficiency F0806
(Tag F0806)
Could have caused harm · This affected 1 resident
**NOTE- TERMS IN BRACKETS HAVE BEEN EDITED TO PROTECT CONFIDENTIALITY** Based on observations, interviews and record reviews, the facility failed to provide food that meets residents' preferences, for...
Read full inspector narrative →
**NOTE- TERMS IN BRACKETS HAVE BEEN EDITED TO PROTECT CONFIDENTIALITY** Based on observations, interviews and record reviews, the facility failed to provide food that meets residents' preferences, for 2 out of 6 sampled residents observed during dining (Resident #66, Resident #57).
The findings included:
1. A record review showed that Resident #66 was admitted to the facility on [DATE] and readmitted on [DATE] with diagnosis of unspecified Atrial Fibrillation and Hyperlipidemia. The Minimum Data Set (MDS) assessment end of PPS Part A Stay dated 01/09/2025 revealed the resident's Brief Interview of Mental Status (BIMS) score is 11, which indicates moderate cognitive impairment.
During an observation conducted on 03/18/2025 at 12:05 PM this surveyor observed that Resident #66's meal ticket consisted of: Ground and/or soft cooked, soup [NAME] jour, ground corned beef with broth, soft cooked parslied potatoes without skin, soft cooked buttered cabbage, Jello cubes, dinner roll, margarine, condiments and add side mashed potatoes. Resident #66's tray did not have mashed potatoes, and the soft cooked parslied potatoes had the skin on it. Resident #66 was seen eating the potatoes and peeling them with her teeth. Resident #66 looked very annoyed and stated that she did not want the skin on her potatoes.
During an observation conducted on 03/20/2025 at 12:21 PM this surveyor observed that Resident #66's meal ticket consisted of: Ground and/or soft cooked, soup [NAME] jour, ground beef cubes in gravy, rice with vegetables broth, soft cooked green beans, mandarin oranges, dinner roll, margarine, condiments and add side mashed potatoes. Resident #66's tray did not have ground beef cubes in gravy.
2. A record review showed that Resident #57 was admitted to the facility on [DATE] with diagnosis of Osteomyelitis and Type II Diabetes Mellitus without complications. The Minimum Data Set (MDS) Quarterly assessment review dated 11/08/2024 revealed that the resident's Brief Interview of Mental Status (BIMS) score is 15, which indicates no cognitive impairment.
During an observation conducted on 03/20/2025 at 12:15 PM this surveyor observed that Resident #57's meal ticket consisted of soup the day, ground beef tips, steamed rice, green beans, juice packed mandarin oranges, dinner roll, margarine, sugar substitute, 2 peppers, a large salad with chicken on the side, diet coke, add Kens salad dressing with salads. Resident #66's tray did not have the large salad with chicken nor the Kens salad dressing.
In an interview conducted on 03/20/2025 at 2:00 PM the dietary manager/director of food services stated that she has 2 checkpoints, the first one is when they receive the food from the cook and the final checkpoint is in the kitchen when placing the plate on the cart. These two checkpoints are responsible for making the meal ticket match the tray. The residents' preferences are placed in the preferences form.
CONCERN
(E)
Potential for Harm - no one hurt, but risky conditions existed
Deficiency F0805
(Tag F0805)
Could have caused harm · This affected multiple residents
**NOTE- TERMS IN BRACKETS HAVE BEEN EDITED TO PROTECT CONFIDENTIALITY** 4) Review of Resident #175's clinical record documented an admission to the facility on [DATE] with no readmissions. The residen...
Read full inspector narrative →
**NOTE- TERMS IN BRACKETS HAVE BEEN EDITED TO PROTECT CONFIDENTIALITY** 4) Review of Resident #175's clinical record documented an admission to the facility on [DATE] with no readmissions. The resident's diagnoses included End Stage Renal Disease and Anxiety Disorder. Review of Resident #175's MDS admission assessment in progress dated 03/13/25 documented a BIMS score of 13, indicating the resident had no cognition impairment.
Review of Resident #175's care plan titled [resident name] is at risk for malnutrition and noted with low BMI (body mass index) and impaired skin. Therapeutic/mechanical altered diet in place initiated on 03/11/25 with interventions to include NAS (no added salt) Pureed diet.
On 03/17/25 at 12:26 PM, observation of the Masada's Unit in-room dining was conducted. Observation revealed Resident #175 received a pureed diet. The resident pureed meat had moderate amount of loose puree consistency with clear orange liquid pooling around other food items in the plate. Subsequently, an interview was conducted with the resident who stated he will not eat the rest of the food and asked to remove the tray. The resident had an approximately 25% intake. Resident #175's meal ticket documented Pureed Corned Beef (Photographic Evidence Obtained).
On 03/18/25 at 12:39 PM, observation of Resident #175's lunch tray revealed a pureed diet. The resident's pureed meat had a moderate amount of loose pureed consistency with clear orange/brownish liquid pooling around other food items in the plate. (Photographic Evidence Obtained).
5) Review of Resident #77's clinical record documented an admission to the facility on [DATE] with no readmissions. The resident's diagnoses included Cerebrovascular Disease, Cerebral Infarction and Anxiety Disorder. Resident #77's MDS quarterly assessment dated [DATE] documented a Brief Interview of the Mental Status (BIMS) score of 5, indicating the resident had severe cognition impairment.
Review of Resident #77's care plan titled [resident name] is at risk for malnutrition . initiated on 011/28/22 revised on 04/21/24 with interventions to include regular puree diet.
On 03/17/25 at 12:35 PM, observation revealed Resident #77 in bed eating lunch. The resident had a pureed diet. The pureed meat had moderate amount of loose puree consistency with clear orange liquid pooling around other food items in the plate. Observation revealed the resident poured sugar over the pureed meat and stated it had no flavor and proceeded to eat it. Subsequently, an interview was conducted with Resident #77 who stated she will not eat the rest of the other pureed items. Resident #77's meal ticket documented Pureed Corned Beef. (Photographic Evidence Obtained).
On 03/18/25 at 12:36 PM, observation of Resident #77's lunch tray revealed a Pureed diet. The resident's pureed meat had a moderate amount of loose puree consistency with clear orange/brownish liquid pooling around other food items in the plate. Resident #77's meal ticket documented Pureed Hamburger. (Photographic Evidence Obtained).
Based on observations, interviews and record reviews, the facility failed to provide the correct diet consistency for pureed diets for 2 of 3 visits to the main kitchen which has the potential to affect 8 residents on pureed diets and for 3 of 3 sampled residents (Resident #47, Resident #175, Resident #77). Who consume pureed diets.
The findings included:
A review of the facility's policy titled, Pureed - Dysphagia Level 1 showed the following: The pureed consistency is planned according to the Regular consistency, but the texture is modified to a smooth, pudding-like, lump free, pureed consistency texture for all food items. This consistency follows the guidelines set forth by the National Dysphagia Task Force.
1) During an observation conducted on 03/18/2025 at 11:45 AM of the pureed lunch meal on the tray line in the kitchen, the menu consisted of #10 scoop of pureed Hamburger, #8 scoop of pureed cooked vegetables, #8 scoop of pureed Cinnamon Apple, #16 scoop of pureed bread, Garnish of Ketchup and mustard, and condiments. A closer observation of the pureed hamburger revealed a grainy like consistency and the pureed vegetables revealed a lumpy like consistency.
2) During an observation conducted on 03/19/2025 at 11:44 AM of the pureed lunch meal on the tray line in the kitchen, the menu consisted of 6 oz of pureed soup of the day, #8 scoop pureed roasted turkey, #8 scoop pureed gravy, #8 scoop of pureed spinach with onions, ½ cup of pureed fruit cup, #16 scoop of pureed dinner roll. The surveyor sampled all pureed foods provided by the Dietary Manager, and it was noted that the pureed turkey was not smooth and small pieces of turkey was identified.
3) A record review showed that Resident #47 was admitted to the facility on [DATE] and readmitted on [DATE] with diagnosis of hemiplegia and hemiparesis following cerebral infarction affecting right dominant side and other sequelae following unspecified cerebrovascular disease. The Minimum Data Set (MDS) assessment significant change dated 02/07/2025 revealed that the Brief Interview of Mental Status (BIMS) score of 12, which indicates mild to moderate cognitive impairment.
During an observation conducted on 03/17/2025 at 12:09 PM in the main dining room, the pureed roll was observed lumpy with a grainy consistency. Resident #47's meal ticket consisted of Nectar Thick Pureed Soup [NAME] Jour, Pureed Corned Beef, Mashed Potatoes, Pureed Buttered Cabbage, Applesauce, Pureed Roll, Margarine and Sugar substitute, Salt, and Pepper, which matched the meal tray.
During an interview conducted on 03/18/2025 at 3:20 PM the Registered Dietitian stated that they use Source Tech as their guide. The Registered Dietitian further stated that a pureed diet should be very soft like baby food, a mashed potato consistency with no lumps. It should look like a scoop and not runny. The plate should have an appeal.
During an interview conducted on 03/19/2025 at 4:00PM the Speech therapist stated that she has been working in the facility for 6 months. She further stated that pureed food should have the consistency of mashed potatoes-like, no lumps or clumps. They follow the Source Tech guidelines. She further said that a pureed food should look presentable in solid form but smooth enough to swallow.
CONCERN
(E)
Potential for Harm - no one hurt, but risky conditions existed
Infection Control
(Tag F0880)
Could have caused harm · This affected multiple residents
**NOTE- TERMS IN BRACKETS HAVE BEEN EDITED TO PROTECT CONFIDENTIALITY** Based on record review, observations and interviews, the facility failed to follow infection control guidelines for residents on...
Read full inspector narrative →
**NOTE- TERMS IN BRACKETS HAVE BEEN EDITED TO PROTECT CONFIDENTIALITY** Based on record review, observations and interviews, the facility failed to follow infection control guidelines for residents on enhanced barrier precautions for 4 of 21 sampled residents reviewed for Enhanced Barrier Precautions (Resident #39, #46, #103 and #175); and failed to follow infection control practices during dialysis treatments for 2 of 3 sampled residents reviewed for Dialysis (Resident #276 and #278).
The findings included:
Review of the facility's policy titled Enhanced Barrier Precautions undated documented .EBP (enhanced barrier precautions) are indicated during high contact care activities for residents .who has a chronic wound and/or indwelling medical device. High contact resident care activities include dressing, bathing/showering, transferring, .providing hygiene, changing linens .wound care .
1)Review of Resident #103's clinical record documented an initial admission on [DATE] and a discharge to a local hospital on [DATE]. Resident's diagnoses included Ventricular Premature Depolarization, Gallbladder Calculus and Hypertension.
Review of Resident #103's MDS Quarterly assessment dated 01/18/ documented a Brief Interview of Mental Status (BIMS) was not conducted due to the resident is rarely/never understood.
Review of Resident #103's care plan title [resident's name] has a risk for injury/infection r/t (related to) presence of indwelling catheter secondary to a dx (diagnosis) of neurogenic bladder initiated on 10/11/24. Interventions did not include following Enhanced Barrier Precautions.
On 03/18/25 at 8:42 AM, observation revealed Resident #103 in bed and Staff P, CNA repositioning, and rearranging the residents cover sheet. Staff P was not wearing a gown. Observation revealed a urinary drainage bag with no privacy pouch. Consequently, an interview was conducted with Staff P who stated the resident had a foley catheter. Attempted to interview the resident who kept his eyes open and fixed looking at the surveyor and did not answer any questions asked.
On 03/18/25 at 8:46 AM, observation revealed the Infection Preventionist and Staff M, UM placing a cart with Personal Protective Equipment outside Resident #103's room. Consequently, an interview was conducted with Staff M and the Infection Preventionist who they both stated the resident had a foley and they will follow EBP (Enhanced Barrier Protection). The Infection Preventionist was asked why the PPE cart was not placed before and stated the resident was moved from another room. Review of Resident #103's clinical census documented room changed on 01/01/25.
2) Review of Resident #175's clinical record documented an admission on [DATE] with no readmissions. The resident diagnoses included End Stage Renal Disease and Anxiety Disorder.
Review of Resident #175's MDS admission assessment in progress dated 03/13/25 documented a BIMS score of 13 indicating that the resident had no cognition impairment.
Review of Resident #175's care plan titled (resident's name) is on Enhanced-Barrier Precaution r/t (related to) sacral wound and Foley catheter initiated on 03/13/25. Interventions included ENHANCED-BARRIER Precaution: Wear gown and gloves for high-contact resident care activities (such as: dressing, bathing/showering, transferring, changing linens, providing hygiene, changing briefs or assisting with toileting .urinary catheter .wound care (any skin opening requiring a dressing) initiated on 03/13/25.
On 03/17/25 at 12:17 PM, observation revealed Resident #175's room door wide open, and a treatment cart parked in front of the door and the resident's privacy curtain halfway open. The surveyor knocked at the door, and was allowed to enter the room, Staff I stated Staff J was doing the resident's wound. Observation revealed Staff I, CNA and Staff J, Wound Care Nurse (WCN) next to the resident's bedside, the resident had his cover down and was showing a foley tubing and his legs. Staff J stated she was finishing the resident's wound care. Observation revealed Staff J and Staff I pulled the cover sheet and blanket up. Further observation revealed Staff I and Staff J were not wearing a protective gown. Furthermore, observation revealed a Personal Protective Equipment (PPE) cart with gowns and an Enhanced Barrier Precaution (EBP) sign outside the resident's room. Subsequently, a joint interview was conducted with Staff J, WCN who stated Resident #175 had a sacrum stage II pressure ulcer and one midback stage II pressure ulcer.
On 03/19/25 at 8:15 AM, observation revealed a hospice aide at Resident #175's bedside. The hospice CNA was wearing a mask and gloves, but not a protective gown. The resident had a gray color T-shirt on, lower body was uncovered, an adult brief and a foley catheter was observed. Consequently, an interview was conducted with the hospice aide who stated she did the resident upper body and was ready to do the lower body. The hospice aide was asked if she ever wore a gown while taking care of the resident with a foley. The aide stated she had not worn a gown while taking care of Resident #175 and added if the resident was on isolation, she would wear a gown, but not with resident #175.
On 03/20/25 at 10:20 AM, observation revealed an EBP signage and PPE cart by Resident #175's room door.
On 03/20/25 at 10:23 AM, wound care observation for Resident #175 by Staff J, WCN and assisted by Staff I, CNA. Staff J and Staff I entered the resident's room, performed hand hygiene and donned gloves. The staff did don a protective gown. Staff J removed the residents covers, repositioned by pulling the draw sheet, pulled the brief down, removed her gloves, performed hand sanitation, but did not donned a gown. Staff J provided Resident #175's wound care without wearing a gown as required. Staff I assisted Staff J during wound care and did not wear a gown.
On 03/20/25 at 11:05 AM, a joint interview was conducted with Staff I and Staff J. Staff J, WCN was asked why she did not wear a gown during Resident #175's wound care and stated she usually puts a gown on but got distracted. Staff I, CNA stated she was supposed to wear a gown and forgot. Staff I and Staff J were apprised they were observed on 03/17/25 finishing Resident #175's wound care and were not wearing a gown either. Staff J stated they always wear gowns.
3) Review of Resident #46's clinical record documented an admission on [DATE] with a readmission on [DATE]. The resident diagnoses included Unspecified Dementia, Aphasia Following other Cerebrovascular Disease, and Need for Assistance with Personal Care.
Review of Resident #46's Minimum Data Set (MDS) 5 days-admission assessment dated [DATE] documented a Brief Interview of the Mental Status (BIMS) was not conducted due to resident is rarely/never understood indicating the resident had severe cognition impairment. The assessment documented under Functional Abilities and Goals the resident was dependent on the staff to complete the activities of daily living.
Resident #46's active care plan did not include EBP care plan or wound care plan.
Review of Resident #46's physician order dated 03/14/25 documented cleanse left heel blister with NS (normal saline), pat dry, apply betadine moist gauze then cover with dry gauze, wrap with kerlix daily. Physician order dated 02/26/25 documented Wound consult.
Multiple observation from 03/17/25 through 03/19/25 revealed no PPE cart, no EBP signage outside Resident #46's room.
On 03/19/25 at 3:38 PM, observation was conducted of transferring Resident #46 from wheelchair to bed Staff L, LPN and Staff Q, CNA. Staff L and Staff Q donned gloves but did not don a protective gown. Subsequently, an interview was conducted with Staff L who stated the resident had a left heel wound. Observation revealed Staff L removed Resident #46's sock and revealed a dry dressing to the left heel dated 03/18/25.
On 03/20/25 at 10:21 AM, an interview was conducted with Staff O, CNA, who was the regular assigned CNA, stated she did not wear a gown while providing care to Resident #46. Staff O confirmed the resident had a dressing on her heel and the WCN was doing the dressing daily. The EBP signage was reviewed with Staff O who acknowledged she had to wear a gown while providing care to the resident with a wound.
On 03/20/25 at 11:20 AM, an interview was conducted with Staff L, LPN who stated Resident #46 was not on contact precautions and she will not wear a gown during transfer. Consequently, a side-by-side review of EBP signage was conducted with Staff L who acknowledged transferring a resident with a wound requires to wear a gown.
On 03/20/25 at 11:35 AM, an interview was conducted with Staff M, Unit Manager, who was apprised of staff not wearing a gown during high care activities for Residents with wounds and/or foley catheter, Resident #46, 103 and 175.
4. A record review revealed that Resident #276 was admitted to the facility on [DATE] with a diagnosis of End-Stage Renal Disease. The admission Minimum Data Set (MDS) dated [DATE] showed Resident #376 with a BIMS score of 10, which is moderately cognitively impaired. A physician's order dated 03/03/25 for Hemodialysis every Monday, Wednesday, and Friday was also dated 03/03/25.
In an observation conducted on 03/19/25 at 9:44 AM, Staff H Patient Care Technician (PCT) was performing initiation of Central Venous Catheter (CVC) dialysis. She practiced hand hygiene and placed a pair of new gloves to create a clean surface near Resident #276's side table. She then adjusted her face shield and touched the access site without practicing hand hygiene or changing gloves. Staff H removed her gloves, placed another pair without hand hygiene, and continued cleaning the access site. She removed her gloves, cleaned her hands, and put on a new pair of gloves. While connecting the syringes to the access site, she removed her gloves and placed a new pair of gloves without practicing hand hygiene between gloves. She then connected the syringes with the same gloves to the access site.
5. A chart review showed that Resident #278 was admitted to the facility on [DATE] with diagnoses of End Stage Renal Disease and Dependence on Dialysis-an order dated 03/17/25 for Hemodialysis every Monday, Wednesday, and Friday.
In an observation conducted on 03/19/25 at 12:50 PM, Staff H, who was performing a disconnection of CVC on Resident #278. She was observed touching the computer, placing a pair of gloves with no hand hygiene, and proceeded to touch Resident's #278 access site. Staff H removed her gloves, walked over to touch the supply cabinet, and placed a new pair of gloves without practicing hand hygiene before coming back to continue the disconnection of the dialysis.
6. Record review for Resident #39 revealed the resident was originally admitted to the facility on [DATE] with most recent readmission on [DATE] with diagnoses that included in part the following: Heart Failure, Kidney Transplant Status, Open Wound Left Lower Leg Subsequent Encounter. The Minimum Data Set, dated [DATE] documented in Section C a Brief Interview of Mental Status score of 14 indicating a cognitive response.
Review of the Physician's Orders for Resident #39 revealed an order dated 03/13/25 for cleanse right heel wound with normal saline, path dry, apply Betadine, cover with gauze, wrap with kerlix every day shift for wound.
Review of the Physician's Orders for Resident #39 revealed an order dated 03/13/25 for cleanse wound to right leg with normal saline, pat dry, apply betadine, cover with dry protective dressing every day shift for wound.
Review of the Physician's Orders for Resident #39 revealed an order dated 03/18/24 for Enhanced Barrier Precaution for wound care.
Review of the Skin Assessment for Resident #39 dated 03/12/24 documented in part the following: Other (not specified) Right Leg anterior-length:5.8cm-width:2.7cm- depth:0.3cm- -etiology: trauma-stage: N/A-granulation: 80%-drain: serous-amt: mild-TX (Treatment):Santyl daily. Right heel length:4.8cm-width:4cm-depth:0.5cm-etiology: pressure stage: unstageable- granulation: 10%-drain: serous- amt: mild- TX: Santyl daily
On 03/18/25 at 8:55 AM an observation was made of an already in progress wound care being provided for Resident #39 performed by Staff J Wound Care Licensed Practical Nurse who was assisted by Staff I Certified Nursing Assistant (CNA) and both staff members were not wearing a gown. There was no Enhanced Barrier Precaution sign on resident's door, and no isolation cart near the Resident's door.
During an interview conducted on 03/19/25 at 2:50 PM with Staff I Certified Nursing Assistant (CNA) who was asked if she knew what Enhanced Barrier Precautions (EBP) were, she stated it is a type of isolation. When asked what type of requirements are needed for EBP she said it would be any wounds and some other things. When asked how do you know who is on EBP, she said there is a sign on the door and then we can ask the nurse what type of precaution it is. When asked when a resident is on EBP what Personal Protective Equipment (PPE) is needed, she stated a gown and gloves. When asked about wound care provided and assisted on 03/18/25 for Resident #39 and not wearing a gown, she acknowledged there was no sign on the door and there was no isolation cart in front of the resident's room.
During an interview conducted on 03/20/25 at 1:15 PM with Staff J Wound Care Licensed Practical Nurse who stated she has been with the facility for almost 1 year. When asked about EBP when she would wear PPE, she stated she would wear gown and gloves when they have an order for EBP and a sign on the door. When asked if a resident has a wound would they be on EBP she said yes.
CONCERN
(F)
Potential for Harm - no one hurt, but risky conditions existed
Food Safety
(Tag F0812)
Could have caused harm · This affected most or all residents
Based on observation, interview, and record review, the facility failed to store, prepare, distribute and serve food, in accordance with professional standards for food service safety for 1 of 3 visit...
Read full inspector narrative →
Based on observation, interview, and record review, the facility failed to store, prepare, distribute and serve food, in accordance with professional standards for food service safety for 1 of 3 visits to the main kitchen.
The findings included:
A review of the facility ' s policy titled Refrigerators, revised in December 2014, showed the following: The facility will ensure safe refrigerator maintenance, temperatures, and sanitation and will observe food expiration guidelines. The acceptable temperature ranges for refrigerators are 35 degrees Fahrenheit (F) to 40 degrees F.
In a tour of the main kitchen conducted on 03/17/25 at 8:55 AM accompanied by the Dietary Manager, the following concerns were noted:
The walk-in refrigerator on the dairy side had an internal temperature of 49 degrees F and not the necessary 40 degrees F and below.
An egg platter pulled out of the dairy walk-in refrigerator had an internal temperature of 43.7 degrees F, not the necessary 40 degrees F and below.
A tuna platter pulled out of the dairy walk-in refrigerator had an internal temperature of 43.5 degrees F, not the necessary 40 degrees F and below.
A scoop of tuna pulled out of the dairy walk-in refrigerator had an internal temperature of 44 degrees F, not the necessary 40 degrees F and below.
A container of nutritional juice drink from the dairy walk-in refrigerator had an internal temperature of 46.0 degrees F, not the necessary 40 degrees F and below.
Another container of a nutritional juice drink from the dairy walk-in refrigerator had an internal temperature of 47.1 degrees F, rather than the necessary 40 degrees F and below.
A large container of raw chicken exposed was noted in the walk-in meat refrigerator. The date 03/08/25 indicated the date the chicken container was placed in the refrigerator.
A large container of raw meat was noted in the walk-in meat refrigerator. Its date of 03/14/25 indicated the date the meat container was placed in the refrigerator. Closer observation revealed a pool of blood on the bottom of the meat container.
A bag of unidentified meat packet which was dated 03/26/25.
A red bucket was tested using the facility Hydrion strips, which showed a level of 400 parts per million, indicating that too much sanitation solution was used in the red bucket. In this observation, the Dietary Manager acknowledged that too much solution was placed in the red bucket.
The dry storage room was noted with a dented can of sliced pineapples that was not placed on the side with do not use sign.
The dry storage room was noted with two dented cans of tomato sauce that were not placed on the side with do not use sign.
An opened bottle of extra light amber honey was half-used in the dry storage area, and the date of its opening is unknown.
A personal 20-ounce Styrofoam cup of coffee was noted in the food production area.
A large metal container noted with a dried unidentified substance coating the surface of the metal container.